Brazil is a vibrant market packed with opportunities. Markem-Image is meeting the needs of our customers by combining our traditional expertise with investment in new technology writes Julio Leite, Markem-Imaje’s Regional Sales Director for Brazil and Argentina.
Systech technology allows manufacturers to add a digital layer of protection to their products that is impossible to counterfeit. As it is based on an already existing mark, our innovative solution is non-additive, representing substantial cost savings.LUX Global Label is a leading manufacturer of pressure-sensitive labels, shrink sleeves and security solutions whose customers include many of the world’s most trusted brands. In 2020, LUX initiated a highly fruitful collaboration with Systech...
When Spanish wire manufacturers Cables RCT identified opportunities for improvement in its complex marking and coding processes, it turned to Markem-Imaje for solutions.
Wellness! It’s the consumer word of our times and the noun that has sold a thousand products: from vitamin supplements to fitness products and snacks. Whilst the shift over the last decade to personal wellness and hypervigilance of what we put in our bodies is well documented, less well known is the growing trend for wellness in pet products.
Right now, one of the most notable consumer trends is the growing appetite for fresher produce with greater supply chain transparency. This is putting pressure on producers and suppliers to identify innovative yet cost-effective ways to package and code directly at the point of harvest.
When you start a company, your goal is to create a popular and profitable product. You are decidedly not thinking about the fact that your product will get counterfeited and sent to the gray market when it does get popular. And trust me, that will happen.When I met with the American skincare brandOZNaturals® they were riding the wave of rapid growth and impressive success. The company was launched in 2013 by CEO Angela Irish with the goal of creating a range of clean, efficacious, and...
Blue Bite’s RevOps Director Jon Anderson explains how to transform products into a digital platform to engage consumers in ways current channels cannot.
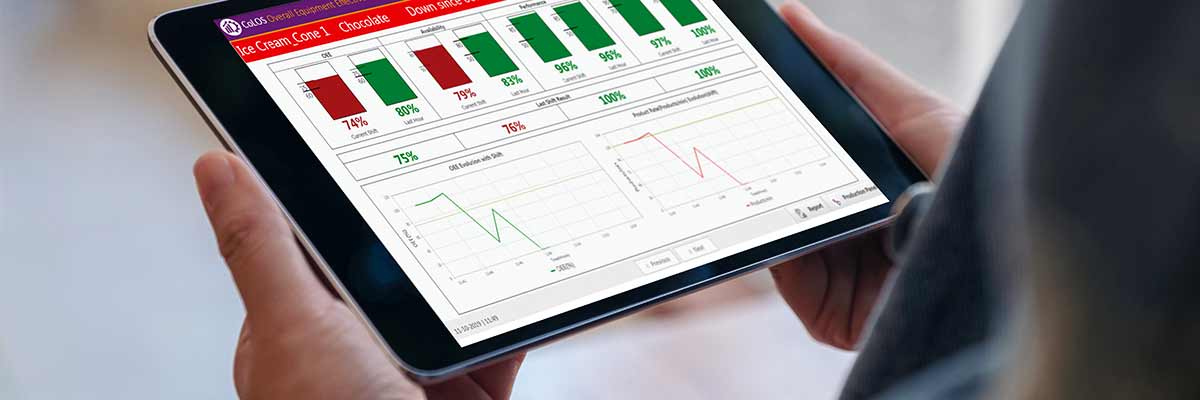
Plant managers are regularly challenged to increase throughput on production lines. Improvements to existing infrastructure or processes is often the best option, but this requires knowing which changes to make. This is the aim of overall equipment effectiveness (OEE): a best-practices metric that indicates how well a manufacturing operation is utilized compared to its full potential. The OEE method measures line productivity based on three key performance indicators: 100% availability (no stop...
Manufacturers need to make sure that marking is correct and readable to ensure compliance with regulations, transparency for customers and to minimize product rework, scrap and recalls. We talk to David Habib, Markem-Imaje’s North America Solution Architect for Packaging Integrity for an expert’s view.