Now let’s look at some of the complications for drug manufactures when meeting the Europe's Falsified Medicines Directive (FMD), and particularly the Delegated Regulation.
The opioid crisis in the United States has been described as an epidemic requiring urgent action on the part of government, law enforcement, health advocates and other stakeholders. A substantial portion of overdose deaths result from opioids produced by reputable manufacturers for legitimate medical purposes that have been redirected to illegitimate use. This transfer of a legal substance to illegitimate or illicit use is known as diversion, and represents a major challenge in the...
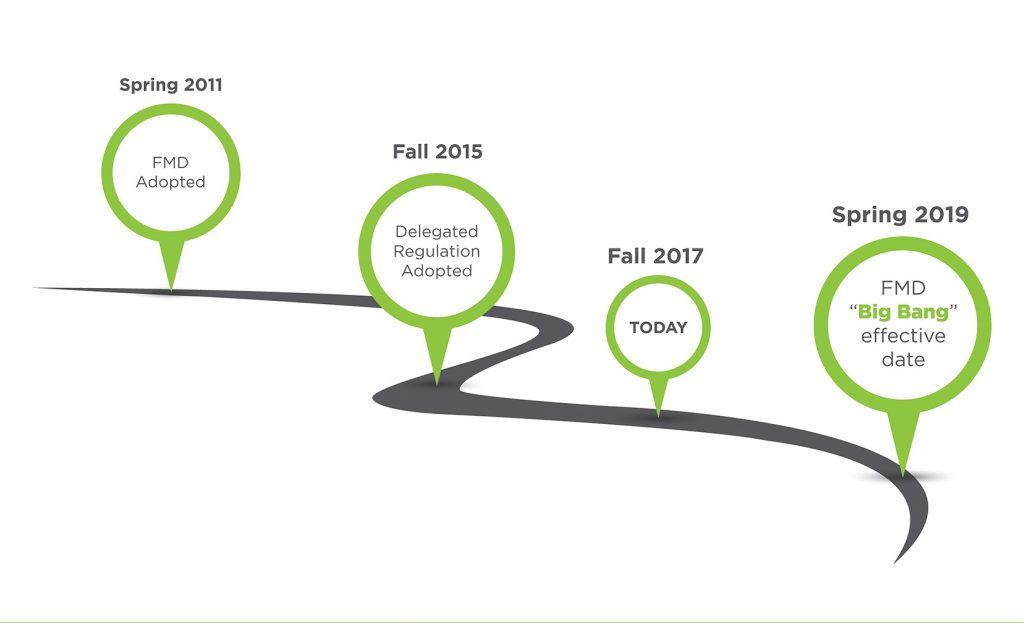
Companies who market prescription drugs in any part of Europe should pay close attention to the new requirements spelled out by the Falsified Medicines Directive (FMD) and Delegated Regulation (DR). The FMD/DR spell out new and complex requirements for applying randomized unique identifiers to almost all prescription drug packages marketed in the EU by February 9, 2019. That date is now less than 18 months away. And it’s not just the EU Member States because other European countries have...
Advisory firm KPMG recently conducted a detailed survey of executives from companies within a technology supply chain to find out how gray market trading was impacting them. The report and its findings are applicable in many commercial supply chains; and so, we recommend you download it from KPMG.
There is a lot of talk right now surrounding the upcoming solar eclipse happening on August 21, 2017, which will be primarily visible across the United States. However, the conversation is less about the eclipse and more focused on Amazon’s recent recall on several “suspect” eclipse glasses that were purchased through the eCommerce giant’s retailers. These “suspect” glasses, as they are being referenced, do not adhere with the ISO standard governing them. While Amazon has not released the names...
Aggregation is a technique used in conjunction with serialization that establishes and records the relationship between a serialized object and its container, which also is serialized. Aggregation is most frequently used in the supply chain to place smaller packaging units inside of larger ones, such as cartons packed into cases and cases onto pallets. The aggregation relationship directly mirrors the physical packing of the objects into their container.
In a recent article, International Pharmaceutical Industry made the point that Serialization Improves Public Health. We agree with that, up to a point. The authors point out that drugs pass “…through a complex distribution network where [their] authenticity at every level cannot be checked due to the absence of data-sharing systems.” Later in in the article they say, “…in the end serialization will help pharmaceutical companies to ensure patient safety.” And, “…reliable serialization and...